Abstract
Background:
JAK2 mutations almost always accompany World Health Organization (WHO)-defined polycythemia vera (PV) (Blood 2016;127:2391); about 97% of these are JAK2 V617F exon 14 mutations while the remainder 3% harbor other JAK2 mutations, of which JAK2 exon 12 mutations are by far the most frequent (Leukemia 2007;21:1960). Mouse models and clinical studies have both revealed phenotypic differences between JAK2 exon 12 and JAK2 V617F mutated PV; the former was characterized by erythroid-dominant myeloproliferation and younger age (( N Engl J Med 2007; 356:459 ; Blood 2016; 128:839 ; Blood 2011 ; 117 : 2813 ) . The main objective of the current study was to identify consecutive cases of JAK2 exon 12 mutated PV from two centers of excellence in myeloproliferative neoplasms and compare their natural history with their JAK2 V617F mutated counterparts.
Methods:
Diagnoses were according to WHO criteria (Blood 2016;127:2391). JAK2 exon 12 mutation analysis was performed by Sanger sequencing of exons 12-15 or next generation sequencing (NGS). Statistical analyses considered clinical and laboratory data collected at the time of initial diagnosis. A separate Mayo Clinic cohort of PV patients with JAK2 V617F mutation, controlled for time of diagnosis (1989-2016), was used for comparison of phenotype, overall (OS), leukemia-free (LFS), myelofibrosis-free (MFFS) and thrombosis-free (TFS) survival data.
Results:
JAK2 exon 12 mutation variants and their frequencies:
Mutation variants (frequency) among all 33 consecutive PV cases with JAK2 exon 12 mutations (21 from Mayo and 12 from Florence) included: H538-K539delins (n=8; 24%), F537-K539delins (n=6; 18%), N542-E543delins (n=5; 15%), E543-D544del (n=5; 15%), K539L (n=4; 12%), R541-E543delins (n=2; 6%) and one each of I540-E540delins, H538-I540del and F532IK540I.
Comparison of phenotype between JAK2 exon 12 and JAK2V617F mutated PV
Significant differences, between JAK2 exon 12 (n=33) and JAK2 V617F mutated (n=364) patients, were noted for median age (54 vs 62 years; p=0.02), median platelet count (281 vs 434 x 10(9)/L; p=0.0003) and median leukocyte count (8.4 vs 11.4 x 10(9)/L; p=0.0008), respectively. We observed no difference in median hemoglobin level (17.3 vs 18.1 g/dL; p=0.08), prevalence of palpable splenomegaly (27% vs 24%; p=0.66), abnormal karyotype (17% vs 20%; p=0.8) or history of thrombosis at time of diagnosis (18% vs 28%; p=0.2); similarly, the incidence of all thrombotic events (27% vs 42%; p=0.1), arterial events (15% vs 28%; p=0.1) or venous events (12% vs 18%; p=0.4), before or after diagnosis, was similar between the two groups.
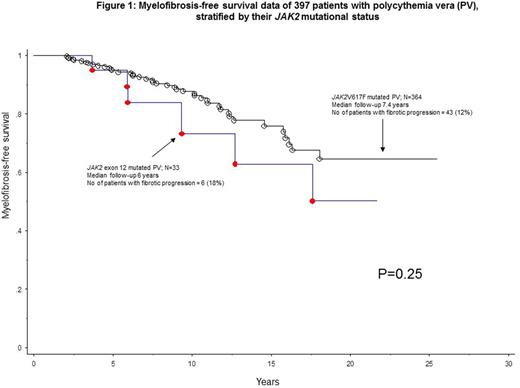
Comparison of OS, LFS, MFFS and TFS between JAK2 exon 12 and JAK2V617F mutated PV
Consistent with the study design that required the comparator cohort (JAK2 V617F mutated PV) be selected based on similar time range for initial diagnosis, there was not significant difference (p=0.3) in median follow-up for JAK2 exon 12 (6 years; range 0-23) vs JAK2 V617F mutated patients (7.4 years). During this period, the number (%) of deaths, leukemic transformations, fibrotic progressions and post-diagnosis thrombotic events, among JAK2 exon 12 vs JAK2 V617F mutated patients were 2 (6%) vs 121 (33%), 0 vs 12 (3.3%), 6 (18%) vs 43 (12%), and 4 (12%) vs 76 (19.5% of evaluable cases), respectively; of note, the 6 fibrotic progressions in JAK2 exon 12 mutated PV occurred in 2 (40%) of 5 patients with E543-D544del, 2 (33%) of 6 patients with F537-K539delins and 2 (25%) of 8 patients with H538-K539delins variants. Head-to-head comparison revealed no significant difference in MMFS (p=0.25; Figure 1) or TFS (p=0.32), between JAK2 exon 12 and V617F mutated disease; furthermore, an apparent difference in OS (p=0.02) was no longer evident when analysis was adjusted for age (p=0.01) or leukocyte count (p=0.08).
Conclusions: The current study provides long-term outcome data on JAK2 exon 12 mutated PV, which appear to be similar to those of JAK2 V617F mutated disease. Furthermore, we confirm and expand on previously published phenotypic correlations.
Vannucchi: Novartis: Honoraria, Speakers Bureau; Shire: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal